According to the Centers for Disease Control (CDC) publication, Preventing Chronic Disease, a rare disease in the United States is one that affects less than 200,000 Americans. In Europe, in order to be considered “rare”, a disease can affect no more than 1 out of every 2,000 people.
Top Stats
- In 2013, 400 million people worldwide were living with a rare disease.
- At least 7,000 rare diseases have been discovered.
- Since the Orphan Drug Act was passed in 1983, 600 new drugs have been developed for rare diseases.
- Advances in medicine have reduced measles and rubella incidences from affecting around 15,000,000 Americans at their peak to affecting fewer than 1,000 today.
In 2013, the total number of people affected by the various types of rare diseases in the U.S. was 25 million. That number was even higher in Europe, with 30 million people affected by various rare diseases, and the total global number skyrocketed to 400 million people, as visualized in the chart below.
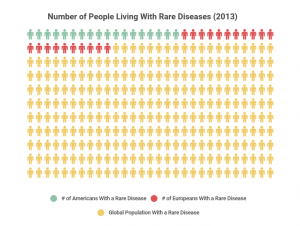
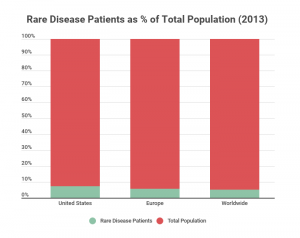
It’s estimated that there are over 7,000 rare diseases worldwide. In 2015, about 5% of the global population was living with a rare disease. New rare diseases continue to be discovered, and it’s often hard to pin down exact numbers because rare diseases can easily be misdiagnosed as something else.
Almost all genetic diseases are considered rare diseases because they are limited to people with certain genes. In fact, 80% of rare diseases are caused by abnormal genes. However, rare diseases can also include certain types of cancers and some infectious diseases, as well.
And finally, some rare diseases aren’t contracted until adulthood or aren’t identified until adulthood, so it’s not just children who are impacted. Although, 1 out of 2 (50%) of rare disease patients are children — and 3 out of 10 (30%) will not reach their 5th birthday.
Common, or Rare?
Some rare diseases seem fairly common because we’ve heard of them before, either because someone famous has spotlighted the disease, or because we or our loved ones were diagnosed with one—but they are still considered rare based on the number of people who actually contract the disease.
A few of these “well-known” rare diseases in the United States include Lou Gehrig’s Disease (ALS), cystic fibrosis, and sickle-cell disease.
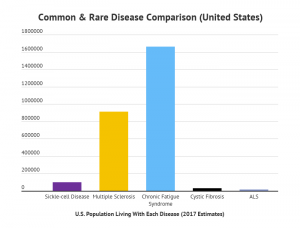
As the chart above shows, Multiple Sclerosis and Chronic Fatigue Syndrome are too prevalent in the U.S. population to fall into the rare disease category, but many people have the mistaken impression that they affect a much smaller segment of the population than they actually do.
Some common diseases have rare types of that disease. So while all types of one disease combined could make a disease common, there may be several rare diseases within the broader umbrella. For instance, cancer is a common disease, but pancreatic cancer is a rare type of cancer, and would, therefore, be considered a rare disease.
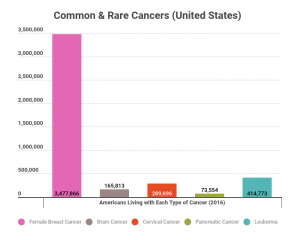
According to the data above, breast cancer is considered a common cancer, since nearly 3.5 million women were living with the disease in 2016. Leukemia and cervical cancers are common, while brain and pancreatic cancers are rare since fewer than 200,000 Americans were living with each type in 2016.
However, it should be noted that there is more than one type of leukemia, and some leukemias are rare diseases.
Can a Disease Change Its Status?
Of course, a disease’s status as rare can change if, as detection and awareness improve, more people end up being diagnosed, as long as the number of people affected by the disease tops the 200,000 mark in the United States, or goes above 1 in every 2,000 people in Europe.
On the other hand, a disease can be rare in one part of the world, and quite common in another. For example, malaria is very common in Africa, but rare in the United States.
Two formerly common diseases are now rare or non-existent in the United States because of advances in available medicines and a greater understanding of how the disease spreads and operates.
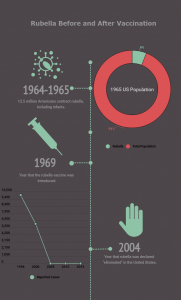
In 1965, more than 12 million Americans contracted rubella, before a rubella vaccine was made available. After introducing a vaccine in 1969, rubella cases declined until the disease was declared eliminated in the United States in 2004. In the past 15 years, fewer than ten Americans each year have contracted rubella.
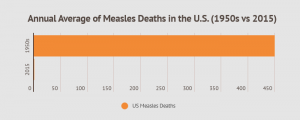
According to estimates, 3 to 4 million Americans contracted measles each year in the 1950s. Of those, there were between 400 and 500 deaths and 48,000 hospitalizations annually. Another 1,000 Americans developed swelling on the brain each year from the virus. After a vaccine was introduced in the 1960s, and decades of widespread vaccinations, there were only 372 reported cases of measles in the U.S. in 2018.
Is “Rare” the Same as “Fatal”?
The severity of each disease varies. Some rare diseases are fatal, though there are variations in expected life span depending on the disease. For some, the prognosis is only a few years into childhood, while for others, the disease may allow them to live into middle age or longer.
Other rare diseases are only life-threatening if the correct treatment isn’t given, or isn’t given quickly enough, to counteract the worst symptoms.
Still, others are not fatal at all but can impact growth and development or quality of life.
Orphan What?
Rare diseases have also been called “orphan diseases,” typically because there aren’t many people in the medical or pharmaceutical communities willing to “adopt” them for research.
It was often believed that it wasn’t worth a medical researcher’s or pharmaceutical company’s limited time and resources to work to understand a disease that affects a relatively small number of people.
For example, in 2015, more than 30 million Americans were living with diabetes. When looking at a disease that only impacts a few thousand people, it can begin to seem like the costs of pursuing some type of action would outweigh any potential benefits.
That’s where the Orphan Drug Act comes into play. All drugs in the United States are subject to approval and oversight by the Food and Drug Administration (FDA). The Orphan Drug Act was passed in 1983 to encourage companies to develop new drugs for rare diseases, setting out guidelines for submitting new drug approval requests to the FDA and opening the door for clinical trials of experimental drugs or biologics for rare diseases.
According to the Act, an orphan drug is one specifically intended to be used in the treatment of a rare disease. Incentives, including grants, tax credits, and exclusive right to market the drug for seven years, were made available through the law to entice entities to work on the development for new drugs, medical devices, and biologic treatments for rare diseases, as it was noted that the perceived cost was a known barrier to developing orphan drugs.
Currently, only about 5% of rare diseases have an FDA-approved treatment. To combat this, there are over 450 million new medicines in development for rare diseases. Although, 50% of rare diseases do not have a specific foundation supporting or researching them.
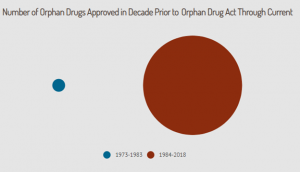
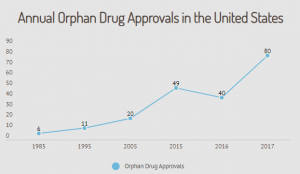
Since the Orphan Drug Act was enacted over 35 years ago, more than 600 drug and biologic products designated for rare diseases have come to market. From 1973 to 1983, the decade prior to the Act, only ten drugs for rare diseases came to market. If the production of orphan drugs had continued at the pre-Act rate, then only 45 new orphan drugs would have been produced between 1983 and 2018.
Here is just a tiny sampling of orphan drugs that have come to market through the Orphan Drug Act (brand names are used where known for ease of recognition). The selection of the drugs included in this list is random and is not intended as an endorsement of any brand or manufacturer.
Rather, the list is designed to shine a light on the variety and reach of available products that have been introduced as a result of the Act.
- Abthrax, Anthim, Anthrasil: Three different drugs that have all been designated for the treatment of anthrax.
- Ascor L 500: An injectable ascorbic acid for the treatment of scurvy when oral treatments are ineffective or impractical.
- Valstar: Used in the treatment of certain types of bladder cancers.
- Crysvita: Approved to help treat X-linked hypophosphatemia in patients one year old or older.
- Genotropin: Long-term pediatric treatment for patients with Prader-Willi syndrome.
- Proleukin: Approved for treating adults with ocular melanoma.
- Provigil and Xyrem®: Two different drugs approved for treating different symptoms of narcolepsy.
- Avastin and Gliodel®: Two different drugs approved for the treatment of glioblastoma.
A Diagnosis for Every Disease?
Even with the huge leaps in the field of knowledge about rare diseases, that doesn’t mean that every rare disease has a “solution” or even a diagnosis. Some conditions are so rare that they can’t be connected to anything else out there, or are so multifaceted that it’s unclear whether the rare disease patient’s symptoms are caused by a new, previously unknown disease, or caused by one or more common diseases acting in concert.
There are no easy answers when it comes to rare diseases, but research, genetic tests, and even social networking (by comparing stories and experiences) are beginning to fill in some of the gaps. Does that mean that every medical mystery will have an answer? Not necessarily. But there are more people around the globe focusing on rare disease research than ever before.
Where Can I Learn More?
There are multiple resources online with information about rare diseases. A few of them are included here. This is by no means an all-inclusive list, and is not intended as an endorsement of any group, organization, or agency.
If you know the name of the rare disease you want more information on, Orphanet has global data in a searchable database on everything from active clinical trials to current research about the disease to characteristics and symptoms of that disease. Information is available in a wide variety of languages, not just English.
Another helpful online resource is Global Genes. This non-profit organization was founded to help those who have been diagnosed with rare diseases and their loved ones. They have information on support groups, rare diseases, clinical trials, and more.
The National Organization for Rare Disorders (NORD) has broken out its website into subsections by user type: rare disease patients and families, patient organizations, and medical clinicians and researchers. The site also has a section on how to become involved in advocacy. It is mainly focused on American users.
Rare Diseases Europe offers a section on orphan drugs on their website, and also includes sections that address policy, provide access to training, and of course, include information on various rare diseases and available treatments. The site is also accessible in the major European languages, including French, Spanish, Italian, Russian, German, and Portuguese.
Although it’s not limited to just rare diseases, the Center for Disease Control (CDC) compiles data in the United States on reported rare diseases and their prevalence. They have quick fact sheets about various diseases and links to research articles and publications that provide in-depth information on several rare diseases.
Another U.S. government agency with facts on rare diseases is the National Cancer Institute. Their Surveillance, Epidemiology, and End Results program (SEER) website is a repository for cancer information and statistics, including rare forms of cancer. This site offers access to studies and other technical data.
Many families and rare disease patients have found that online support and advocacy groups are safe places to share their experiences and journeys to find a diagnosis. Several of the websites mentioned above have links to various advocacy and support groups on their websites.
The Research Effect
We wanted to wrap up this article with a few examples of the powerful impact that additional resources, new medications, and advanced research have had in the field of rare diseases.
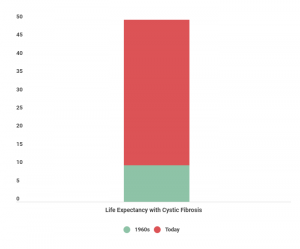
Cystic fibrosis is a genetic disease that mainly affects the lungs, pancreas and liver. Once, patients diagnosed with cystic fibrosis only had a life expectancy of ten years of age. Through improvements in care, new drugs and treatments that didn’t exist a few decades ago, the majority of people with this disease now have a life expectancy of at least 40 years of age.
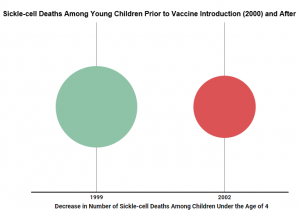
Sickle-cell disease impacts red blood cells, making them hard and misshapen. Death rates for children under the age of four who were born with sickle-cell disease dropped by 42 percent between 1999 and 2002, due in part to a vaccine that was introduced in 2000.
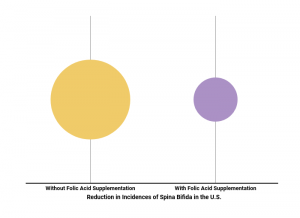
Spina bifida is a failure of the spinal column to close all the way. Before antibiotics were widely available, most babies born with the most serious form of the disease died before they could receive treatment. Thanks to improved surgical procedures and treatment with antibiotics, 90 percent of children born with spina bifida survive.
Studies done in the 1990s showed that taking folic acid before and during the early stages of pregnancy reduced the chances of spina bifida by 70 percent.
In Summary…
There are many resources out there to help those who have been diagnosed with rare diseases. The ones named in this article are a great place to start and check out your specific state’s website for any resources that may be locally available. Rare Disease Day is also a great time to raise awareness in the community.
More breakthroughs are being made every day, and with hospitals like the Mayo Clinic doing clinical trials and research on a massive scale, worldwide rare-disease databases compiling statistics from around the globe, and organizations like the CDC providing education to medical professionals, rare diseases are no longer shrouded in a fog of mystery and misinformation.
Sources
- United States Census Bureau, “U.S. and World Population Clock.”
- Centers for Disease Control and Prevention (CDC), “Public Health and Rare Diseases: Oxymoron No More.”
- Orphanet, “About Rare Diseases.”
- Worldometers, “World Population by Year.”
- Eurostat, “EU28 Population 505.7 Million at 1 January 2013.”
- ALS Association, “What is ALS?”
- CDC, “Data & Statistics on Sickle Cell Disease.”
- Cystic Fibrosis Foundation, “About Cystic Fibrosis.”
- National Multiple Sclerosis Society, “Landmark Study Estimates Nearly 1 Million in U.S. Have Multiple Sclerosis.”
- CDC, “ME/CFS: Making Strides to Enhance the Lives of Those Living with ME/CFS.”
- National Cancer Institute, “Cancer Stat Facts: Female Breast Cancer.”
- National Cancer Institute, “Cancer Stat Facts: Brain and Other Nervous System Cancer.”
- National Cancer Institute, “Cancer Stat Facts: Pancreatic Cancer.”
- National Cancer Institute, “Cancer Stat Facts: Leukemia.”
- National Cancer Institute, “Cancer Stat Facts: Cervical Cancer.”
- CDC, “About Malaria.”
- CDC, “Malaria’s Impact Worldwide.”
- CDC, “The Pink Book: Rubella.”
- CDC, “Rubella in the U.S.”
- CDC, “Stop Rubella – Make Sure Every Child Gets the Rubella Vaccine.”
- Google, “Population in the U.S.”
- CDC, “Measles History.”
- CDC, “Measles Data and Statistics.”
- CDC, “Measles Cases and Outbreaks.”
- Forbes, “First Confirmed Measles Death in More Than a Decade.”
- CNN, “Measles Rarely Kills in the U.S. – But When it Does, Here’s How.”
- U.S. Food and Drug Administration, “Developing Products for Rare Diseases and Conditions.”
- Electronic Code of Federal Regulations, Title 21, Chapter 1, Subchapter D, Part 316, “Orphan Drugs.”
- Public Law 97-414
- U.S. Department of Health and Human Services, “List of FDA Orphan Drugs.”
- IQVIA™, “Orphan Drugs in the United States (Part One).”
- CDC, “New CDC Report: More Than 100 Million Americans Have Diabetes or Prediabetes.”
- CDC, “What is Sickle Cell Disease?”
- Emedicinehealth, “Spina Bifida.”
- Christopher and Dana Reeve Foundation, “Spina Bifida.”
